Pharmaceutical
Small Polar Molecules
Comprehensive analysis, including inorganics and chromatographically challenging compounds.
Simple analysis of Small Polar molecules
Analysis of polar and reactive species – such as formaldehyde, acetaldehyde and formic acid – is slow and complicated when using conventional chromatographic methods. SIFT-MS simplifies detection of small polar species by analyzing them directly from gas or headspace to sub-ppbV concentrations, without requiring derivatization or preconcentration.
Applications of SIFT-MS for analysis of challenging compounds include:
- Residual monomers and byproducts
- Residual solvents in pharmaceuticals
- Packaging impurities
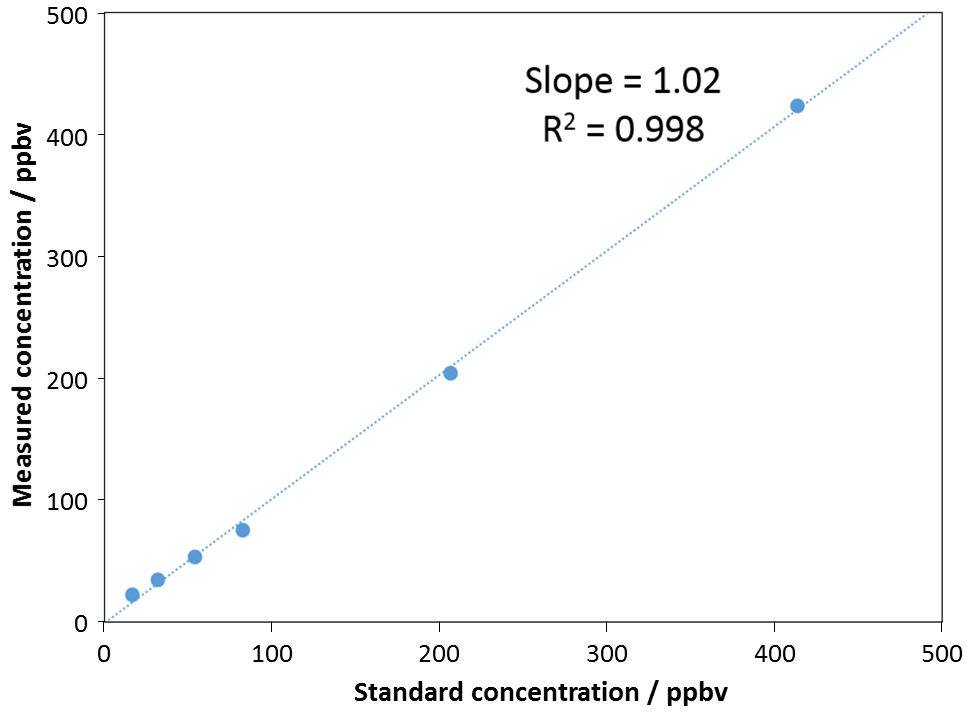
Linear detection of formaldehyde in the ppbV range.
Small Polar Molecules benefits
Comprehensive analysis, including inorganics and chromatographically challenging compounds
Flexible sample delivery options, including automation and area sampling
Intuitive software and fast method development
High sensitivity and wide linearity range
Automated high-throughput headspace analysis
Small Polar Molecules resources

Pharmaceutical Applications
SIFT-MS represents a major breakthrough for the pharmaceutical industry due to its ability to comprehensively analyze diverse VOCs and inorganic gas impurities with very high sample throughput. It quantifies VOCs directly in real-time to sub-part-per-billion (ppb) concentrations, so that product issues are detected earlier and resolved immediately, delivering economic benefits to all stakeholders.
Formaldehyde in Air (sample bags)
Direct analysis using selected ion flow tube mass spectrometry (SIFT-MS) enables real-time monitoring of formaldehyde to sub-part-per-billion concentrations. SIFT-MS simplifies and accelerates both sampling and analysis of formaldehyde, providing 25-fold throughput enhancements.

Rapid, Simplified Residual Solvent and Volatile Impurity Analysis Using SIFT-MS
This webinar describes SIFT-MS applications in the pharmaceutical industry, including:
- Its use as an alternative procedure for USP <467> residual solvent analysis
- Rapid, quantitative analysis of leachable formaldehyde from polyoxymethylene polymer and PEG excipient
- High-throughput headspace screening for nitrosamine residues in drug products
- Fast turnaround, simplified analysis of ethylene oxide residues in detergents used for cleaning validation.


Pharma Method Validation - Formaldehyde
This application note demonstrates that by applying a strategy in accordance with International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Validation of Analytical Procedures: Text and Methodology Q2(R1) Guidelines (or “ICH Q2(R1)”) to direct analysis methods using SIFT-MS, successful validation is readily achieved – even for formaldehyde.

Direct MS Simplifies Analysis of Challenging Compounds
This webcast focuses on case studies that demonstrate simple analysis of chromatographically challenging compounds. Formaldehyde receives special attention, as it is important across a wide range of industries (from environmental to pharmaceutical testing).

Formaldehyde: Real-time, Sensitive Gas and Headspace Analysis Using SIFT-MS
Various exciting applications of SIFT-MS formaldehyde analysis will be presented, including candle flame analysis, material emissions and evaluation of materials that scavenge gaseous formaldehyde.
Drying Endpoint Monitoring Using SIFT-MS For Enhanced Manufacturing Of Active Pharmaceutical Ingredients
Process analysis using SIFT-MS enables the drying process to be monitored past the drying end-point measurable using conventional weighing methods. This delivers greater efficiencies for production, and lowers risk of thermally damaging sensitive APIs.
SIFT-MS Selected Ion Flow Tube Mass Spectrometry
Learn about direct mass spectrometry by SIFT-MS which provides real-time, quantitative analysis of volatile compounds with trace-level sensitivity. There are also no requirements of chromatography, pre-concentration, or sample prep! SIFT-MS is easy to use and interpret data.

Simple, Rapid Analysis Of Ethylene Oxide In A Polysorbate 80 Excipient Using SIFT-MS
Quantitative ethylene oxide analysis in Polysorbate 80 excipient is greatly simplified using SIFT-MS, with a time to first test result that is eight-fold faster than the current compendial method and a daily sample throughput that is 9- to 14-fold higher.
Simple, Rapid Analysis Of Formaldehyde Impurities In Gelucire Excipient Using SIFT-MS
SIFT-MS greatly simplifies formaldehyde detection and quantitation through direct, instantaneous, and sensitive (sub-ppbV) sample ionization, yielding sample throughputs of up to 250+ samples/day.
Simple Rapid Analysis of NDMA in a Recalled Valsartan Product Using SIFT-MS
Quantitative analysis of NDMA impurities in drug products is greatly simplified using SIFT-MS and has a three-fold throughput advantage (excluding sample prep benefits) over chromatographic methods.
Syft Tracer: The Next Generation of Volatile Impurities Analysis for Enhanced Workflows
This app note introduces the next generation of SIFT-MS, Syft TracerTM, which launched at Pittcon 2023. It revolutionizes volatile impurities analysis workflows through unparalleled speed, performance stability, and reproducibility. Learn about how this innovation to real-time trace gas detection outpaces chromatography-based methods in the analysis of challenging analytes such as formaldehyde in a PEG excipient.

The Latest Innovation of Real-Time, High-Throughput Volatile Impurities Analysis by SIFT-MS
Join us for this webinar to learn about Syft Tracer, the latest advancement of real-time, trace gas analysis by SIFT-MS which launched at Pittcon 2023. Hear how the recent product innovations unlock analytical bottlenecks and enable faster decisions to be made in critical process steps.
Revolutionary Productivity For Volatile Residue and Impurity Analysis
This application note describes a scenario in which Syft TracerTM replaces five chromatography systems and still has significant available sample capacity. SIFT-MS provides rapid, chromatography-free analysis that revolutionizes multiple workflows.
Extending SIFT-MS Residual Solvent and Volatile Impurity Analysis to Water-Insoluble Articles
SIFT-MS can analyze residual solvents and other volatile impurities in water-insoluble articles using a two-step process: dissolution in compatible solvent, then dilution in water. This application note describes a systematic evaluation of the compatibility of six organic solvents with headspace-SIFT-MS analysis following this two-step process. Quantitative headspace analysis is achieved at 12 samples/hr, providing significant productivity improvements compared with chromatographic approaches.

Revolutionizing Workflows for Residual Solvents and Volatile Impurities Analyses by SIFT-MS
This webinar demonstrates how the new, automated Syft TracerTM SIFT-MS platform provides a comprehensive solution to workflow challenges and can replace multiple chromatographic systems. Learn about how combining SIFT-MS with automation provides a very flexible and high throughput solution for screening volatile impurities in pharmaceutical and consumer safety applications, revolutionizing workflows.
Revolutionizing Volatile Impurities Analysis Through Next Gen SIFT-MS
This E-book describes how next gen SIFT-MS enables rapid, continuous screening of toxic volatile impurities in pharmaceutical and consumer products. This advancement to SIFT-MS delivers trace-level detection sensitivity, unparalleled performance stability, superior selectivity, and highly reproducible, quantitative data. Never miss a contamination event again.
Improved MHE-SIFT-MS Workflows - Concentration Independent MHE Calibration
This application note investigates concentration dependence of MHE calibration in sample matrix. Across the full range of analytes investigated in this study, MHE calibration holds for at least one order of magnitude change in sample concentration. For analytes in the C7–C9 range, the MHE calibration applies over two orders of magnitude analyte concentration. These results mean that the MHE workflow can be applied to a wider range of samples in the matrix, further reducing calibration demand.
EtO and Acetaldehyde in Polysorbate 80: Rapid Quantitative Analysis Using SIFT-MS
This study evaluates a headspace-SIFT-MS approach for Polysorbate 80 products from multiple suppliers and evaluates ethylene oxide and acetaldehyde recoveries. Results confirm that the headspace-SIFT-MS procedure reliably quantifies these analytes without requiring purification of Polysorbate 80 for matrix-matching the calibration standard. Hence, headspace-SIFT-MS analysis of ethylene oxide and acetaldehyde can revolutionize existing workflows.
Residual Solvent Analysis: Optimization for DMI Solvent
DMI is a very promising non-aqueous solvent because it can be used even without subsequent dilution in water. Using a subset of residual solvents, this study confirms that moderately polar volatiles yield headspace responses that are independent of the volume of sample used. This means that cost and environmental impact can be reduced. Furthermore, with SIFT-MS this optimization can be carried out very rapidly due to direct sample analysis and high sample throughput.
Syft Tracer Pharm11
Learn about the world's only 21 CFR Part 11 compliant real-time mass spec, Syft Tracer Pharm11, which is launching at Contract Pharma and debuting at AAPS 2023 PharmSci 360!
Rapid Analysis of Residual Solvents and Volatile Impurities for High-Throughput CDMO Workflows
In this webinar, we introduce our new compliant real-time mass spectrometer solution, Syft Tracer Pharm11. A single instrument is capable of running up to 220 samples per day, and multiple methods and analyses can be performed in sequence. The direct headspace-SIFT-MS instrument is chromatography-free, and therefore the need for multiple columns and instrument downtime between methods does not exist. We will highlight several common CDMO tests that have been performed and validated using headspace SIFT-MS, including residual solvents, ethylene oxide, nitrosamines and other extractables & leachables.
Faster Quantitative Analysis of Volatile Impurities Using MHE-SIFT-MS
In this application note, enhanced MHE workflows are demonstrated using styrene, formaldehyde, and NDMA analyses in polystyrene polymer, Gelucire excipient, and ranitidine drug products, respectively. Reduced calibration frequency in routine analysis enables significant workflow benefits to be realized, including four-fold faster time to first result for quantitative analysis of condensed-phase samples. Over 220 samples per day can be analyzed quantitatively for diverse volatile impurities using the enhanced MHE-SIFT-MS workflow.

SIFT-MS: Real-Time Volatiles Analysis for Continuous Manufacturing of Pharmaceuticals
Watch this on-demand webinar featuring Mark Perkins (Element), Professor Chris Price (CMAC, University of Strathclyde) and Aaron Smith (CMAC, University of Strathclyde) to learn how SIFT-MS provides real-time analysis of volatile impurities when determining solvent drying end points, volatile degradation products, or volatile impurities during hot melt extrusion.
Accelerating residual solvents analysis in 21 CFR Part 11 compliant settings through real-time mass spectrometry
SIFT-MS is a direct, real-time mass spectrometry (MS) technique which offers revolutionary volatile compound analysis capabilities to Pharma and CDMO labs due to its fast time to data, time efficient workflows, analytical flexibility, and ease of use. It expedites analytical workflows, such as residual solvents analysis, by generating faster results than traditional methods. Syft Tracer Pharm11 is a SIFT-MS-based solution that includes SyftAuditTracer software designed for 21 CFR Part 11 compliant environments. This app note describes how volatile impurities can be characterized in real-time including nitrosamines, ethylene oxide, and residual solvents.
Supporting 21 CFR Part 11 Regulated Workflows with Next Gen SIFT-MS
This white paper describes how SyftAuditTracer software supports compliance with 21 CFR Part 11 regulations in pharma and CDMO environments. SyftAuditTracer is part of the Syft Tracer Pharm11 bundle solution for high-throughput, compliant workflows.
Quantitative Analysis of NDMA in Drug Products
This paper describes a proposed high-throughput method for analyzing nitrosamines in drug product using Headspace–SIFT-MS. In this study, N-nitrosodimethylamine (NDMA) was quantified directly and rapidly from drug product without dissolution, at levels well below the regulatory acceptable intake of 96 ng day−1. Use of the novel MHE-SIFT-MS approach may enable a wider screening of drug products to be conducted, since it provides around a three-fold increase in daily sample throughput.



