
Pharma and CDMO
Real-time VOC analysis for pharma and CDMO applications. SIFT-MS delivers high-throughput solutions that increase your capacity and operational efficiency in residual solvent analysis, nitrosamine characterization, ethylene oxide and impurity detection, and detergent and sterilization monitoring. Perform real-time volatile pharmaceutical impurities analysis with Syft's 21 CFR Part 11 compliant solution, Syft Tracer Pharm11.
SIFT-MS delivers high-throughput solutions that eliminate bottlenecks in development, production, and QA/QC environments.
The real-time data output of SIFT-MS significantly shortens time to data and increases sample throughput. Analytical bottlenecks in pharma and CDMO applications can be overcome, increasing capacity and operational efficiency in applications such as residual solvent analysis, nitrosamine characterization, ethylene oxide and impurity detection, and detergent and sterilization monitoring.
Learn about the world's only 21 CFR Part 11 compliant real-time mass spectrometer that is debuting worldwide! Syft Tracer Pharm11 is a bundle solution for high-throughput, 21 CFR Part 11 compliant, automated workflows in pharmaceutical and CDMO applications. Contact us to schedule a live demo at an upcoming event or in your lab! Read about how it speeds up volatile impurities analysis in compliant settings.
Pharma and CDMO applications and use cases
Small Polar Molecules
SIFT-MS simplifies detection of small polar species by analyzing them directly from gas or headspace to sub-ppbV concentrations, without requiring derivitization or pre-concentration.
Read MoreResidual Solvent Analysis
SIFT-MS provides rapid characterization of compounds that are not easily monitored by other technologies.
Read MoreDrug Delivery Devices
SIFT-MS provides a very rapid and highly sensitive solution for the detection of residual monomers and other impurities in diverse drug delivery devices.
Read MorePackaging Testing
SIFT-MS revolutionizes packaging testing across diverse materials with its ability to analyze a very wide range of compounds rapidly.
Read MoreSyft is your answer in the Pharmaceutical industry

Industry issue
For pharmaceutical companies investing in scale-up of their manufacturing processes, the associated scaling of residual solvent testing using the USP<467> procedure requires significant upscaling of analysis capacities. This involves increased investments in qualified personnel, lab space, validation procedures and maintenance as well as higher overall instrument acquisition and running costs.
Competitive benefit
The validated SIFT-MS procedure meets the acceptance criteria in USP<1467>, and with 17-fold higher sample throughput, addresses current scale-up issues for residual solvent testing in the pharmaceutical industry.
Industry impact
Since SIFT-MS uses direct, chromatography-free analysis, practical issues for the front-end separation are eliminated, resulting in a robust and reliable analytical result that is easily obtained and requires less qualified personnel. Capacity increase can thus be reached with just a single automated SIFT-MS instrument.What a CDMO Analytical Director Says About SIFT-MS
Christopher Williams
"It combines the detectability of small organic compounds with the speed of something like an elemental analysis instrument. It can just cycle through testing very quickly." - Christopher Williams, Director of Development Services, Alcami Corporation
Director of Development Services, Alcami Corporation
Pharma / CDMO Industry
Pharmaceutical resources

Pharmaceutical Applications
SIFT-MS represents a major breakthrough for the pharmaceutical industry due to its ability to comprehensively analyze diverse VOCs and inorganic gas impurities with very high sample throughput. It quantifies VOCs directly in real-time to sub-part-per-billion (ppb) concentrations, so that product issues are detected earlier and resolved immediately, delivering economic benefits to all stakeholders.
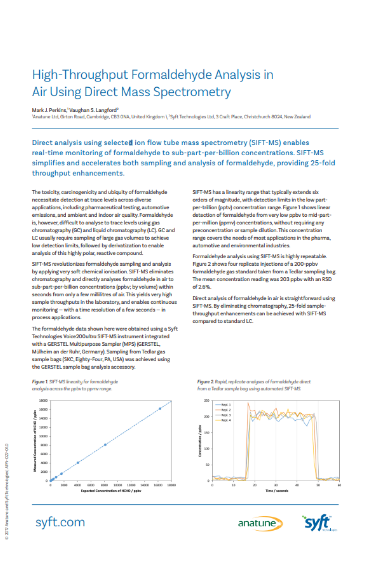
Formaldehyde in Air (sample bags)
Direct analysis using selected ion flow tube mass spectrometry (SIFT-MS) enables real-time monitoring of formaldehyde to sub-part-per-billion concentrations. SIFT-MS simplifies and accelerates both sampling and analysis of formaldehyde, providing 25-fold throughput enhancements.

Rapid Volatile Impurity Analysis in Pharmaceutical Products Using SIFT-MS
SIFT-MS quantifies volatile compounds that are chromatographically challenging such as formaldehyde, formic acid and ammonia. Direct, real-time analysis using SIFT-MS provides new opportunities across multiple pharma applications, including:
- Simple formaldehyde analysis
- Packaging screening, including residual monomer analysis
- Residual solvent analysis
- Cleaning validation
- Production facility air quality monitoring
- Bioreactor monitoring

Rapid, Simplified Residual Solvent and Volatile Impurity Analysis Using SIFT-MS
This webinar describes SIFT-MS applications in the pharmaceutical industry, including:
- Its use as an alternative procedure for USP <467> residual solvent analysis
- Rapid, quantitative analysis of leachable formaldehyde from polyoxymethylene polymer and PEG excipient
- High-throughput headspace screening for nitrosamine residues in drug products
- Fast turnaround, simplified analysis of ethylene oxide residues in detergents used for cleaning validation.

Direct MS Simplifies Analysis of Challenging Compounds
This webcast focuses on case studies that demonstrate simple analysis of chromatographically challenging compounds. Formaldehyde receives special attention, as it is important across a wide range of industries (from environmental to pharmaceutical testing).

Residual Monomer Analysis
In this paper, the unique capabilities of SIFT-MS are illustrated for residual monomer analysis, using formaldehyde emissions from polyoxymethylene (POM) polymer to illustrate. SIFT-MS provides significant throughput increases for both testing laboratories and quality assurance/quality control (QA/QC) in a process environment.

Analysis of Volatile Emissions from Polymers and Paperboard
This webinar describes a recent addition to the portfolio of techniques available for routine sample analysis: SIFT-MS. SIFT-MS enables rapid, quantitative analysis of VOCs across the full spectrum of packaging materials. Detailed case studies will describe applications in polymeric and paperboard emissions analysis.

Drying Endpoint Monitoring Using SIFT-MS For Enhanced Manufacturing Of Active Pharmaceutical Ingredients
Process analysis using SIFT-MS enables the drying process to be monitored past the drying end-point measurable using conventional weighing methods. This delivers greater efficiencies for production, and lowers risk of thermally damaging sensitive APIs.

Simple, Rapid Analysis Of Ethylene Oxide In A Polysorbate 80 Excipient Using SIFT-MS
Quantitative ethylene oxide analysis in Polysorbate 80 excipient is greatly simplified using SIFT-MS, with a time to first test result that is eight-fold faster than the current compendial method and a daily sample throughput that is 9- to 14-fold higher.

Paperboard Volatiles 3 - Odor Rating
SH-SIFT-MS provides an instrument-based odor rating technique for the analysis of diverse odor compounds – such as short-chain organic acids, aldehydes and reduced sulfur compounds. Throughput of at least 12 samples per hour can be achieved using this method.

Volatile Compound Analysis: Automated SIFT-MS
SIFT-MS provides direct, rapid sample analysis of volatile organic compounds (VOCs) with no or minimal sample prep required. When coupled with autosampler technology, SIFT-MS yields very high sample throughput (in excess of 100 samples per hour), including for challenging compounds like ammonia, formaldehyde and hydrogen sulfide. Learn how this direct mass spec technology serves a range of industries, from environmental analysis to food testing to pharma.

Routine Analysis 1: Why Labs and CROs Should Adopt Direct MS
This webinar demonstrates the advantages of applying automated SIFT-MS to volatile compound analysis in diverse matrices: from air to the headspace of polymer, soil or water samples. SIFT-MS is not simply advantageous for throughput reasons; it can also support faster method optimization for conventional methods. We include example data and case studies from a range of application areas ranging from pharmaceutical to environmental analysis.

Routine Analysis 2: Routine Lab Procedures Are Easy to Apply to Direct MS
Learn how you can adopt direct mass spectrometry in your routine analysis workflow. Adaptation of routine procedures and techniques to automated SIFT-MS will be illustrated with case studies applicable to environmental, consumer product and biomedical laboratory testing. In particular, a head-to-head comparison of GC/MS and SIFT-MS for headspace analysis of volatiles in blood plasma will be described.

Routine Analysis 4: Direct MS Simplifies Analysis of Challenging Compounds
This webinar focuses on case studies that demonstrate simple analysis of chromatographically challenging compounds. Formaldehyde receives special attention, as it is important across a wide range of industries (from environmental to pharmaceutical testing). The formaldehyde case study also includes validation of an unconventional analytical method in compliance with ICH Q2(R1) guidelines for regulatory submission.

Syft Application Talks 5: Determination of the Supercritical Carbon Dioxide Extraction End-Point
This webinar discusses an online measurement system for monitoring organic solvent removal of organic solvents during the drying process using SIFT-MS. This method enables real-time, high-resolution analysis of the performance of various conventional and novel drying technologies, with little requirement for analytical method development. Example results and benefits of this approach are discussed.

Part 4: Biomedical and Pharma
In this webinar, we describe the wide range of biomedical and pharmaceutical applications in which SIFT-MS solves difficult analytical challenges. These include:
- Breath research, for which there is a significant academic literature
- Analysis of body fluid headspace
- Microbial VOC analysis
- Emerging small-molecule applications for the pharma industry

Online Demonstration: Pharmaceutical Applications
This workshop includes:
- An overview of the current industries that are using SIFT-MS and the companies that are benefiting from it
- What distinguishes SIFT-MS from other mass spectrometry techniques
- Where and how SIFT-MS applies in the pharmaceutical industry
- Live demonstrations: using SIFT-MS to analyze various samples
- Breakdown of sample analysis, including Syft Technologies' custom software that displays data in real-time.

Pharmaceutical Method Validation for SIFT-MS
This webinar describes how SIFT-MS works, how it speeds up analysis and how it has been validated successfully in accordance with ICH Q2(R1) guidelines suitable for pharmaceutical applications, such as regulatory submissions where GMP compliance is essential. This is illustrated by utilizing formaldehyde analysis as a case study.

Syft App Talks #17. Time-Resolved Thermal Extraction of Volatiles from Plastic Materials using TD-SIFT-MS
In this presentation, examples of real-time thermal extraction and analysis of various plastic materials using TD-SIFT-MS are demonstrated, and its application to product development and processing environments are discussed. Direct, continuous analysis of formaldehyde from polyoxymethylene (POM) is of particular significance.

The SIFT-MS Automation Series: Episode 6 SIFT-MS Automation Past, Present and Future
In this sixth and final episode, Dr Perkins reflects on the automation journey and shares his practical "hot tips" for a wide variety of automated analyses. The webinar (and series!) concludes with a discussion of how automated SIFT-MS is an excellent complementary technique to the conventional laboratory "workhorse" techniques: gas and liquid chromatography.

The SIFT-MS Automation Series: Episode 5 Continuous Headspace Analysis… and Beyond and R&D laboratories.
In this fifth episode, Dr Perkins describes the novel continuous headspace analysis (CHA) technique that he developed to measure stripping of volatiles. (Note: CHA is not to be confused with dynamic headspace analysis!). In addition, Mark describes simple, very high-throughput gas sample bag analysis and touches on other recent developments – including thermal desorption-SIFT-MS.

The SIFT-MS Automation Series: Episode 4 Calibration Approaches for Automated SIFT-MS
In this fourth episode, Dr Perkins describes how simply and rapidly traditional instrument calibration is achieved with the automated SIFT-MS configuration. Traditional calibration approaches have not featured regularly in the SIFT-MS literature due to the widespread use of reliable library-based quantitation. However, calibration is essential for contract testing laboratories, so it is addressed in detail and from a number of different angles in this webinar.

The SIFT-MS Automation Series, Episode 3: Advanced Headspace Methods with SIFT-MS
In this third episode, Dr Perkins describes the application of SIFT-MS to multiple headspace extraction (MHE). You will learn how to determine matrix-independent concentrations from MHE. Furthermore, Dr Perkins describes how the speed of SIFT-MS analysis can be leveraged to accelerate method development for both SIFT-MS and conventional methods – for example, through rapid determination of headspace equilibration times.

The SIFT-MS Automation Series, Episode 2: Accelerate Static Headspace Analysis with SIFT-MS
In this second episode, Dr Perkins provides a practical guide to automated static headspace (SH) analysis with SIFT-MS. You will learn how SH-SIFT-MS can address throughput challenges and simplify analysis of chromatographically challenging species.

The SIFT-MS Automation Series, Episode 1: Why Automate Analysis?
In this first episode, Dr Perkins answers the important question:
Why Automate Analysis?
This webinar introduces the benefits of automation and discusses how they can be applied to SIFT-MS, enabling straightforward adoption by the contract laboratory.
SIFT-MS Method Validation Webinar
Mark describes how the ICH Q 2 (R1) guidelines can be readily applied to SIFT-MS methods, whether in scenarios where only the analytical technique is changed or for tasks that pose a unique analytical challenge. He presents case studies for two such scenarios: 1) methanolic extraction of BTEX compounds (benzene, toluene, ethylbenzene plus xylenes) from soil, 2) direct analysis of formaldehyde leaching into single-use pharmaceutical devices from polymeric components.
Simple, Rapid Analysis Of N-Nitrosodimethylamine (NDMA) Impurity In Ranitidine Products Using SIFT-MS
Quantitative analysis of volatile nitrosamine impurities in drug products is greatly simplified using SIFT-MS and has a three-fold throughput advantage (excluding sample prep benefits) over chromatographic methods.
Simple, Rapid Analysis Of Formaldehyde Impurities In Gelucire Excipient Using SIFT-MS
SIFT-MS greatly simplifies formaldehyde detection and quantitation through direct, instantaneous, and sensitive (sub-ppbV) sample ionization, yielding sample throughputs of up to 250+ samples/day.

Rapid Residual Solvent Analysis Validation Of An Alternative Procedure For USP Method <467> Using SIFT-MS
This study demonstrates that SIFT-MS provides an alternative procedure to USP<467>. Because SIFT-MS is inherently a rapid test technique (all sample components are simultaneously analyzed in about one minute per sample), this validated procedure can be used in organizations that require high-throughput testing, providing 17-fold daily throughput increase over GC-FID.
Simple Rapid Analysis of NDMA in a Recalled Valsartan Product Using SIFT-MS
Quantitative analysis of NDMA impurities in drug products is greatly simplified using SIFT-MS and has a three-fold throughput advantage (excluding sample prep benefits) over chromatographic methods.
High-Throughput, Quantitative Analysis of Benzene in Personal Care Products using Headspace-SIFT-MS
SIFT-MS provides rapid, sensitive, and robust analysis of benzene and other contaminants in a wide array of commercial products. Integration with a headspace autosampler enables rapid screening of hundreds of samples per day.
Real-Time Monitoring of Bioreactor Production Processes Using SIFT-MS
The ability of SIFT-MS to monitor critical volatile organic compounds (VOCs) produced by a bioreactor in real-time at low parts-per-billion by volume (ppbV) concentrations is demonstrated. This application note presents the results of a pilot-scale feasibility study conducted over several weeks, demonstrating the utility of SIFT-MS for maximizing production outcomes.
A Consumer Safety Expert Shares His Experience with SIFT-MS
We asked David Light, CEO and Founder of Valisure, about his experience using SIFT-MS and how it impacted their dry shampoo safety study.

Feedback from a CDMO SIFT-MS User
Hear what Christopher Williams, Director of Development Services, Alcami Corporation, had to say about his experience using SIFT-MS.
Rapid Screening of Paperboard For Volatile Leachables Using Static Headspace-SIFT-MS
Automated SIFT-MS analysis rapidly screens paperboard packaging for a wide range of functional groups in a single analysis, assuring that the volatile emissions meet manufacturer specifications. This application note describes a Headspace-SIFT-MS method with throughput >10X faster than traditional GC/MS methods.
Rapid, Quantitative Analysis of Volatile Aldehyde Impurities In Paperboard Using Multiple Headspace Extraction
Quantitative determination of volatile aldehyde residues in paperboard is readily achieved using multiple headspace extraction-SIFT-MS analysis, with an eight-fold throughput increase over the equivalent conventional analysis.
Rapid Odor Screening of Paperboard Using Static Headspace-SIFT-MS
Automated SIFT-MS analysis coupled with multivariate statistical analysis provides rapid sensory screening of paperboard packaging, overcoming the low sample throughput of sensory panels. The ability to use HS-SIFT-MS to achieve throughput of 12 samples per hour is demonstrated.

Rapid, Simplified Residual Solvent and Volatile Impurity Analysis Using SIFT-MS
SIFT-MS provides real-time, selective, and economic analysis of challenging volatile compounds, such as residual solvents, formaldehyde, dimethylamine, and NDMA without requiring derivatization or other special handling.

High-Throughput Analysis of Volatile Impurities in Sustainable Packaging using SIFT-MS
Pharmaceutical and food products are susceptible to contamination from packaging, whether from polymeric materials, printing inks, or paperboard. SIFT-MS, a high-sensitivity direct-injection mass spectrometry technique, enables risks associated with sustainable packaging alternatives to be mitigated through economical, high-throughput automated analysis (typically a 4- to 15-fold increase over GC-MS).
High-Throughput Quantitative Analysis of Residual Monomer by Using MHE-SIFT-MS to Calibrate Single Headspace Injections
MHE-SIFT-MS provides rapid, matrix-independent, quantitative determination of volatile leachables in polymers and calibrates quantitative analysis based on single headspace injections. This approach enables over 200 samples per day to be analyzed, delivering rapid and economic analysis of volatile leachables such as residual monomers.
Syft Tracer: The Next Generation of Volatile Impurities Analysis for Enhanced Workflows
This app note introduces the next generation of SIFT-MS, Syft TracerTM, which launched at Pittcon 2023. It revolutionizes volatile impurities analysis workflows through unparalleled speed, performance stability, and reproducibility. Learn about how this innovation to real-time trace gas detection outpaces chromatography-based methods in the analysis of challenging analytes such as formaldehyde in a PEG excipient.
Head to Head Comparison of Class 2A And 2B Residual Solvents Analysis Using Sift-MS And GC-FID
This application note describes head-to-head comparison of GC-FID and SIFT-MS analyses of Class 2A and 2B residual solvents. The techniques perform similarly for linearity and repeatability, but SIFT-MS provides superior performance for accuracy and recovery. Furthermore, SIFT-MS provides greater than 11-fold increase in sample throughput and significantly reduces the time taken to report quantitative results (over six times faster for a full calibration set).
Recent developments and applications of selected ion flow tube mass spectrometry (SIFT‐MS)
SIFT‐MS is now recognized as the most versatile analytical technique for the identification and quantification of trace gases down to the parts‐per‐trillion by volume, pptv, range. This statement is supported by the wide reach of its applications, from real‐time analysis, obviating sample collection of very humid exhaled breath, to its adoption in industrial scenarios for air quality monitoring. This review touches on the recent extensions to the underpinning ion chemistry kinetics library and the alternative challenge of using nitrogen carrier gas instead of helium.

The Latest Innovation of Real-Time, High-Throughput Volatile Impurities Analysis by SIFT-MS
Join us for this webinar to learn about Syft Tracer, the latest advancement of real-time, trace gas analysis by SIFT-MS which launched at Pittcon 2023. Hear how the recent product innovations unlock analytical bottlenecks and enable faster decisions to be made in critical process steps.
Syft Tracer Brochure
Syft TracerTM is the latest advancement in real-time, direct injection mass spectrometry (MS) built to solve the most difficult analytical challenges faced within a variety of industries and applications. This advancement to SIFT-MS delivers trace-level detection sensitivity, unparalleled performance stability, superior selectivity, and highly reproducible, quantitative data. Syft Tracer is optimized for high-throughput environments where continuous operation is the standard. Never miss a product or environmental contamination event again.
Even Faster Quantitation Of Formaldehyde In Gelucire Excipient
This application note describes how the efficiency of a MHE workflow can be significantly improved for MHE-SIFT-MS due to the stability of the technique. Formaldehyde impurity is analyzed easily and quantitatively in Gelucire excipient with this improved approach. The time-to-result is reduced to 85 minutes for this system – six-fold faster than the conventional MHE-SIFT-MS approach. Quantitative analysis is achieved at the throughput of 220 samples/day.
Revolutionary Productivity For Volatile Residue and Impurity Analysis
This application note describes a scenario in which Syft TracerTM replaces five chromatography systems and still has significant available sample capacity. SIFT-MS provides rapid, chromatography-free analysis that revolutionizes multiple workflows.
Application Brief: Revolutionary Workflows for Volatile Impurities Analysis
This application brief summarizes a scenario relevant to contract research organization (CRO) and contract drug manufacturing organization (CDMO) laboratories where multiple volatile impurity methods need to be conducted in short runs. Five analyses are considered that can be handled by one Syft Tracer but require multiple legacy, chromatography instruments. For these analyses, SIFT-MS reports the first quantitative results 2- to 12-fold faster, and has sample throughputs 3- to 17-fold higher than the conventional procedures.
Headspace-SIFT-MS: Flexibility that Revolutionizes Workflows for Diverse Samples
The characteristic flexibility, stability, high throughput, and fast time to data of the Syft Tracer next-gen SIFT-MS instrument apply across multiple headspace approaches for diverse matrices. This application note briefly summarizes the use of (1) dissolution, (2) multiple headspace extraction (MHE), and (3) the method of standard additions, then provides a guide for identifying the appropriate headspace approach for various matrices.
Extending SIFT-MS Residual Solvent and Volatile Impurity Analysis to Water-Insoluble Articles
SIFT-MS can analyze residual solvents and other volatile impurities in water-insoluble articles using a two-step process: dissolution in compatible solvent, then dilution in water. This application note describes a systematic evaluation of the compatibility of six organic solvents with headspace-SIFT-MS analysis following this two-step process. Quantitative headspace analysis is achieved at 12 samples/hr, providing significant productivity improvements compared with chromatographic approaches.

Revolutionizing Workflows for Residual Solvents and Volatile Impurities Analyses by SIFT-MS
This webinar demonstrates how the new, automated Syft TracerTM SIFT-MS platform provides a comprehensive solution to workflow challenges and can replace multiple chromatographic systems. Learn about how combining SIFT-MS with automation provides a very flexible and high throughput solution for screening volatile impurities in pharmaceutical and consumer safety applications, revolutionizing workflows.
Rapid Sensory Analysis of Paper Packaging Using SIFT-MS
This application note demonstrates that SIFT-MS effectively classifies paper samples by odor intensity rating and odor descriptor, in addition to distinguishing paper composition and mill of origin. When integrated with autosamplers, SIFT-MS provides throughputs of over 220 samples per day, supporting significantly enhanced quality assurance compared to conventional human sensory panels and gas chromatography-based analyses.
Pharmaceutical Residual Solvent Analysis: A Comparison of GC-FID and SIFT-MS Performance
This study expands upon the previous work by conducting a head-to-head comparison of GC-flame ionization detection (GC-FID) and SIFT-MS procedures. SIFT-MS analyzed samples over 11-fold faster than GC-FID, increasing daily sample throughput and reducing the time taken to determine the result. The results suggests that residual solvent analysis using SIFT-MS may support workflow improvements for pharmaceutical manufacturers.
Revolutionizing Volatile Impurities Analysis Through Next Gen SIFT-MS
This E-book describes how next gen SIFT-MS enables rapid, continuous screening of toxic volatile impurities in pharmaceutical and consumer products. This advancement to SIFT-MS delivers trace-level detection sensitivity, unparalleled performance stability, superior selectivity, and highly reproducible, quantitative data. Never miss a contamination event again.
Improved MHE-SIFT-MS Workflows - Concentration Independent MHE Calibration
This application note investigates concentration dependence of MHE calibration in sample matrix. Across the full range of analytes investigated in this study, MHE calibration holds for at least one order of magnitude change in sample concentration. For analytes in the C7–C9 range, the MHE calibration applies over two orders of magnitude analyte concentration. These results mean that the MHE workflow can be applied to a wider range of samples in the matrix, further reducing calibration demand.
Real-Time Analysis of Volatile Emissions from Hot-Melt Extrusion using Untargeted SIFT-MS
SIFT-MS provides unique capabilities that enable formulators to optimize the hot melt extrusion processes by minimizing polymer, API, and other excipient degradation. SIFT-MS provides real-time feedback though online, ultra-trace, gas-phase analysis of the small molecules emitted when the mixture is extruded, benefiting process development and optimization, as well as continuous process monitoring and control.
High-Throughput Screening of Polypropylene Polymer using Untargeted Headspace-SIFT-MS Analysis
This application note describes how SIFT-MS coupled with multivariate statistical analysis can be utilized for rapid screening of pelleted virgin and recycled polypropylene using an untargeted “fingerprinting” approach. Automated headspace-SIFT-MS analysis can classify and qualify polypropylene samples at throughputs of over 230 samples per day, offering great potential for rapid screening of recycled polypropylene feedstock for FCM applications. Adoption of SIFT-MS supports product quality and enhances sustainability.
EtO and Acetaldehyde in Polysorbate 80: Rapid Quantitative Analysis Using SIFT-MS
This study evaluates a headspace-SIFT-MS approach for Polysorbate 80 products from multiple suppliers and evaluates ethylene oxide and acetaldehyde recoveries. Results confirm that the headspace-SIFT-MS procedure reliably quantifies these analytes without requiring purification of Polysorbate 80 for matrix-matching the calibration standard. Hence, headspace-SIFT-MS analysis of ethylene oxide and acetaldehyde can revolutionize existing workflows.
Residual Solvent Analysis: Optimization for DMI Solvent
DMI is a very promising non-aqueous solvent because it can be used even without subsequent dilution in water. Using a subset of residual solvents, this study confirms that moderately polar volatiles yield headspace responses that are independent of the volume of sample used. This means that cost and environmental impact can be reduced. Furthermore, with SIFT-MS this optimization can be carried out very rapidly due to direct sample analysis and high sample throughput.
Syft Tracer Pharm11
Learn about the world's only 21 CFR Part 11 compliant real-time mass spec, Syft Tracer Pharm11, which is launching at Contract Pharma and debuting at AAPS 2023 PharmSci 360!
Rapid Analysis of Residual Solvents and Volatile Impurities for High-Throughput CDMO Workflows
In this webinar, we introduce our new compliant real-time mass spectrometer solution, Syft Tracer Pharm11. A single instrument is capable of running up to 220 samples per day, and multiple methods and analyses can be performed in sequence. The direct headspace-SIFT-MS instrument is chromatography-free, and therefore the need for multiple columns and instrument downtime between methods does not exist. We will highlight several common CDMO tests that have been performed and validated using headspace SIFT-MS, including residual solvents, ethylene oxide, nitrosamines and other extractables & leachables.
Faster Quantitative Analysis of Volatile Impurities Using MHE-SIFT-MS
In this application note, enhanced MHE workflows are demonstrated using styrene, formaldehyde, and NDMA analyses in polystyrene polymer, Gelucire excipient, and ranitidine drug products, respectively. Reduced calibration frequency in routine analysis enables significant workflow benefits to be realized, including four-fold faster time to first result for quantitative analysis of condensed-phase samples. Over 220 samples per day can be analyzed quantitatively for diverse volatile impurities using the enhanced MHE-SIFT-MS workflow.

SIFT-MS: Real-Time Volatiles Analysis for Continuous Manufacturing of Pharmaceuticals
Watch this on-demand webinar featuring Mark Perkins (Element), Professor Chris Price (CMAC, University of Strathclyde) and Aaron Smith (CMAC, University of Strathclyde) to learn how SIFT-MS provides real-time analysis of volatile impurities when determining solvent drying end points, volatile degradation products, or volatile impurities during hot melt extrusion.
Accelerating residual solvents analysis in 21 CFR Part 11 compliant settings through real-time mass spectrometry
SIFT-MS is a direct, real-time mass spectrometry (MS) technique which offers revolutionary volatile compound analysis capabilities to Pharma and CDMO labs due to its fast time to data, time efficient workflows, analytical flexibility, and ease of use. It expedites analytical workflows, such as residual solvents analysis, by generating faster results than traditional methods. Syft Tracer Pharm11 is a SIFT-MS-based solution that includes SyftAuditTracer software designed for 21 CFR Part 11 compliant environments. This app note describes how volatile impurities can be characterized in real-time including nitrosamines, ethylene oxide, and residual solvents.
Supporting 21 CFR Part 11 Regulated Workflows with Next Gen SIFT-MS
This white paper describes how SyftAuditTracer software supports compliance with 21 CFR Part 11 regulations in pharma and CDMO environments. SyftAuditTracer is part of the Syft Tracer Pharm11 bundle solution for high-throughput, compliant workflows.
Quantitative Analysis of NDMA in Drug Products
This paper describes a proposed high-throughput method for analyzing nitrosamines in drug product using Headspace–SIFT-MS. In this study, N-nitrosodimethylamine (NDMA) was quantified directly and rapidly from drug product without dissolution, at levels well below the regulatory acceptable intake of 96 ng day−1. Use of the novel MHE-SIFT-MS approach may enable a wider screening of drug products to be conducted, since it provides around a three-fold increase in daily sample throughput.
High-Throughput Quantitation of NDMA in Recalled Metformin using Headspace-SIFT-MS
This application note describes a headspace-SIFT-MS method for the analysis of N-nitrosodimethylamine (NDMA) that compares well with LC-MS/MS approaches and holds a 3X throughput advantage. Seamless transitions between test methods make Syft Tracer the most efficient and flexible instrument for analysis of volatile impurities, including in QC applications.




